Update 5/2023. CareDx full-year guidance; plans layoffs. Here. Share price is $8, vs $15 six months ago, but most of that was due to a half-price cut around March 1, stable since then.
####
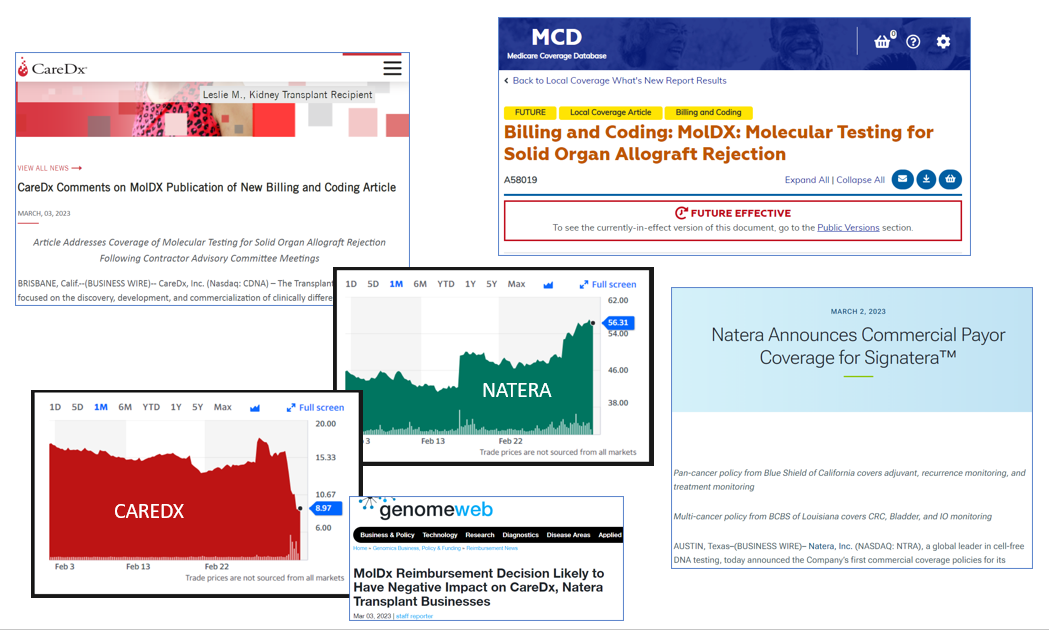
MolDx and other payers move markets. Over a few weeks, Natera is up from $42 to $50 to $56, ending with a market cap of $6.2B. During the same time, CareDx is down from around $16 to $9, ending with a market cap of $480M. Details follow.
Natera Up Again
In a press release March 2, 2023, Natera announces a range of commercial payor coverage for Signatera, a tumor-exome-based minimal residual disease (MRD) test for cancer recurrence. Named plans include Blue Shield of California and BCBS of Louisiana. However, the announcements are also a benchmark for future coverage from other plans on a rolling basis. Ten days earlier, the test had gotten MolDx coverage in breast cancer, here.
As share price has climbed from $42 to $50 to $56, market cap has risen about $1.5B, from around $4.7B to around $6.2B.
CareDx Transplant Documents Revised
There are complicated alterations to statements of policy for the suite of CareDx tests for transplant rejection, and I won't attempt to map all the details in this blog.
(In addition, CareDx refers to its own conversations and Q&A with MolDx, and CareDx has probably made remarks in other investor calls, without aggregating all the details of which, it's impossible to interpret precisely from the new MolDx documents.)
Key documents include:
- CareDx press release on March 3 here.
- The NEW transplant billing article A58019 here. Eff. March 31.
- The Current (now "Old") transplant billing article A58019 here.
- Medicare held a medical-scientific workshop on this area of medicine last year, transcripts are here.
- At the time, I pointed out there was some daylight between the way Palmetto and Noridian described the same meeting - my blog here. In the workshop, some of the statements in the transcript (including from CMS medical directors) seemed skeptical.
- Coverage at "Genomeweb" here, March 3.
Least Helpful "Explanation of Changes" Ever
When they change LCD's and Articles, near the bottom of the document, MACs are required to leave a public explanation of changes (the new one is R6 and has a row for explaining its changes.) CMS doesn't define "explanation," but dictionaries do, such as "a reason or justification given for an action or belief."
In explaining R6, which moved the stock market so much, Medicare writes,
- Article: Added four paragraphs.
- Deleted: Table.
- Group 1: Deleted sentence.
It made be easier to see this in a table:
|
Explanation
|
Explanation of
Changes (Rev 6)
|
|
"A reason or justification given for an action or
belief."
|
Article: Added four paragraphs
|
|
Deleted: Table.
|
|
Deleted: Sentence (section Group 1)
|
The table "deleted" was a detailed 7-row table that provided specific details and conditions for use (for payment) for seven tests, being Prospera (Natera donor DNA), Allosure Kidney, TruGraf, AlloSure Heart, AlloMap, Viracor Trak, and QSant (urine based, w/ DNA). This table is just gone.
- The new four paragraphs provide four major rules for implementing the transplant LCD, which I clip as "Appendix - 4 Rules."
A key deleted sentence required AlloMap and AlloSure to be billed together if AlloSure was billed.\ This is replaced by a sentence stating, "For a given patient encounter, only one molecular test for assessing allograft status may be billed."
In a follow-up email, MolDx noted that the additions (I'm calling them "rules" for shorthand, there are 4) are all available from a close reading of one or another part of the text of the original LCD.
MolDx closes by remarking, "Additional Articles may be added to provide more specific billing and coding instruction for specific services." So, with regard to key points, all we can say is, "to be continued."
Pro's and Con's of "Foundational LCDs"
MolDx has shifted to "foundational LCDs" (also for hereditary cancer testing, and for minimal residual disease testing in all cancers and indications) - which allow specific coverage to be updated by articles or other decisions on a rolling basis, without the time-consuming rules for public notice and public comment. So, for example, breast cancer can be added as a Natera MRD indication, quickly, if MolDx decides its data now justifies coverage under principles in the LCD. However, the policy's implementation can also be changed quickly in other ways - as CareDx found out - for example, by deleting the sentence, "AlloSure Heart is to be billed in conjunction with AlloMap."
APPENDIX - Four Rules
The new four rules created for the upcoming version of A58019.
Article Text
The information in this article contains billing, coding or other guidelines that complement the Local Coverage Determination (LCD) for MolDX: Molecular Testing for Solid Organ Allograft Rejection L38568.
NOTES:
RULE ONE
-For a given patient encounter, only one molecular test for assessing allograft status may be billed. Any additional molecular tests billed after the first will be denied and subject to medical review.
RULE TWO
-Different Z-Code identifiers must be used for protocol vs for-cause testing.
NOTE that use of the molecular test for surveillance (protocol) testing is only compliant with the policy if the patient is enrolled at a center that utilizes this practice and would otherwise receive a surveillance (protocol) biopsy.
Providers must demonstrate that such a practice (for protocol biopsies) is in place to meet coverage criteria of this policy.
RULE THREE
-Performing this test is not compliant with the language of the policy if used for cause [transplant signs and symptoms of problems - bq] when it will not be performed in lieu of a biopsy or to further inform on the need for or results of a biopsy. As such, the test and the biopsy cannot be performed simultaneously or within a short window of time such that the test cannot reasonably inform medical management.
Tests performed within a week AFTER a biopsy are not compliant with policy.
RULE FOUR
-Per policy, one of the intended uses of the molecular test is "For further evaluation of allograft status for the probability of allograft rejection after a physician-assessed pretest."
A pre-test is defined here as "other physiologic/laboratory/clinical evidence consistent with rejection."
Therefore, performing the molecular test at the same time as the pre-test is NOT compliant with the policy. The results of the pre-test must be available to the treating clinician to inform the need for a molecular test or biopsy.
[For example, the patient has elevated creatinine on Monday (pre test) and gets molecular test on Wednesday. - BQ]
###
Frequency.
I was originally puzzled that the LCD didn't say much explicitly about "frequency." However, on re-reading this week, it's probably not so vague. If the test is used instead of a protocol biopsy, it would be the same frequency (let's say, quarterly in Year 1). So that's clear enough. If the test is used for cause - signs and symptoms of rejection - it would be used because of the clinical decline of the patient (elevated creatinine, decreased urine output, fever, etc). So that's probably clear enough, too.
###
CareDx shares popped from $14.70 to $17.80 on the Tuesday the 28th, with its investor call, then slipped to $9 on March 3, ending with a market cap of $480M. Peak to trough almost exactly 50%.