(Note, this recapitulates in shorter form some of the material in my own Sidebar 3, the FDA LDT supplement.)
###
The FDA press release about the LDT proposal doesn't mention finances at all, except a qualitative remark that "benefits would outweight the costs."
The proposed rule says very little about costs, except for some cryptic summary information at beginning and end. The "annualized" 20 year benefits (the 20 year value divided by 20) will be $2B to $86B per year. The annualized costs will be $2B to $19B per year (the 20 year costs divided by 20).
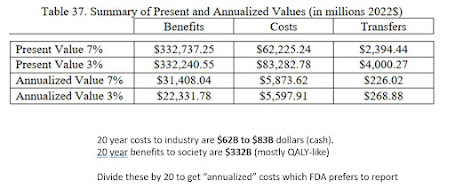
Find footnote 34 of the Rule and it takes you to a 127-page PDF at the FDA, the preliminary financial analysis. Slog through to the end, to find tables 35 and 37. Those are the "money slides." Here's what they say.
Table 35 above shows that benefits begin at $14B per year as soon as LDT registration is put in place [!], rapidly rising to about $26B per year out to year 20.
The benefits, we learn, are mostly driven by value-years of about 10,000 patients living about 3 years longer for a value of about $2M ($2M x 10,000 = $20B). All of those benefit numbers are highly conjectural and reflect implied or intangible values.
It also shows that costs will be $10B and $27B in years 3 and 4, tapering to $3B per year out to year 20.
The costs are actual lab compliance costs. To put that $37B in perspective, the recent annual pre tax earnings of both Labcorp and Quest together are around $2.5B.
In table 37, the 20-year costs are simple added up (with discounting) and then, divided by 20 to annualize them. Thus, the "annualized" cost to industry is about $6B and the annualized benefits to society anywhere from $22B to $31B per year.
What's lost in printing only an annualized 20 year cost is that most of the industry costs are $40-50B in the first few years. (This is also why the present-value costs are much less sensitive to the 3% or 7% discounter).
###
One company recently told Genomeweb it spent over $860,000 on outside resources just trying to get 510(k) clearance for PGx software (here).