UPDATE AUGUST 2023: COVERAGE CANCELED.
https://www.discoveriesinhealthpolicy.com/2023/08/the-lord-given-taketh-away-cms-rescinds.html
####
Annual PSA testing is a statutory benefit for Medicare patients, whether CMS policymakers are thrilled about that or not.
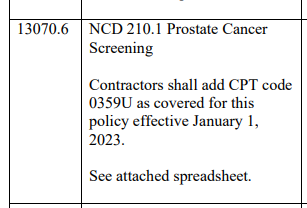
In a relatively obscure publication, in April 2023, CMS added the Cleveland Diagnostics iso PSA test to coverage under the NCD for PSA testing. See transmittal R11952. Item 13070.6. Here.
The addition is interesting because in past years, CMS has either excluded LDTs from coverage under the rules of some NCDs, or even told some stakeholders it will not consider LDTs in its NCD system.
___________________
The code is PLA code 0359U, effective January 2023. The test was under FDA review in 2019 as a breakthrough product, here. Cleveland asserts the test has high sensitivity and 2X better specificity than regular PSA, here. It has a recommendation status at NCCN, here.
The code text is: ONCOLOGY (PROSTATE CANCER), ANALYSIS OF ALL PROSTATE-SPECIFIC ANTIGEN (PSA) STRUCTURAL ISOFORMS BY PHASE SEPARATION AND IMMUNOASSAY, PLASMA, ALGORITHM REPORTS RISK OF CANCER.
For a large study by Klein et al., 2022, here.
Prior to this NCD transmittal, isoPSA was not covered by an NCD so it could not be used as a screening test. It was recently made available when used after a positive "conventional" PSA test - see CGS MAC LCD L39284.
CGS notes that its LCD ahs been initially released as a non coverage draft, and the draft coding would have been 89240. The L39284 LCD became effective just last winter, 11/2022.
##
LDTs and NCDs?
I haven't seen that isoPSA is actually fully FDA approved, so this addition to an NCD seems to violate a rule-in-practice or "unwritten rule" that CMS only considers FDA-approved tests for NCD coverage.
For example, CMS has characteristically written requirements for FDA approval into lab test NCDs. And I've been told CMS won't consider review of the current 2018 CMS NCD for NGS testing in cancer, to allow MRD multiple tests for the first time, until an FDA product appears (for example, an FDA Signatera).
 |
| Not FDA yet? |
 |
| LDT covered by NCD for prostate screening. |
Cleveland Dx raised $19M in early 2021, here.
I noticed a 2022 article, DA59066, that recommended billing isoPSA with code 89240, which would be outdated since the new PLA code is active. Here. See the newer version A59066 from 1/1/2023, here.
Billing is to the CGS MAC, the MAC for Ohio (Cleveland). The style of the long LCD is very similar to the style of the recent MOLDX squamous cancer draft LCD. This suggests that MolDx may have assisted with the analysis, even though the test is proteomic and is not branded as a "MolDx" LCD.
####
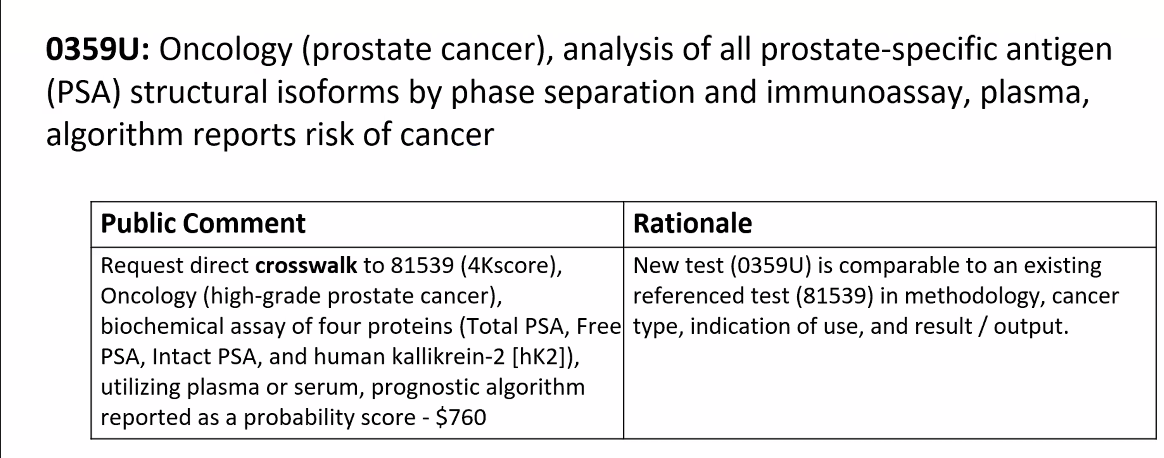
At the June 22, 2023, meeting, IsoPSA requested crosswalk to the 81549 4KScore price, $760
4KScore is an FDA approved test,
https://www.fda.gov/medical-devices/recently-approved-devices/4kscore-test-p190022