SHORT FORM: A new paper on precision oncology in the community proposes pathologists should order tumor testing. However, they don't mention CMS regulatory barriers.
####
On June 20, Precision Medicine Online has a deep dive article by Alison Kanski, discussing an important new study of precision oncology in practice.
- Find the subscription article here.
- Find the original study here.
- Implementation Science Communications, Ellis et al.
- "Determinants of targeted cancer therapy use in community oncology practice."
The study carefully process-mapped 11 cancer care delivery teams via interviews with 24 providers, including oncologists, pathologists, and other staff. The paper takes something of a sociology/anthropology view of roles, processes, conventions, etc. Their main conclusion is reflected in the abstract:
A key determinant of molecular testing was role alignment. The dominant expectation for oncologists to order and interpret genomic tests is at odds with their role as treatment decision-makers’ and pathologists’ typical role to stage tumors. Programs in which pathologists considered genomic test ordering as part of their staging responsibilities reported high and timely testing rates.
Essentially, cancer tumor panels would get ordered more timely and more consistently if this was in the hands of the pathologist who is signing out the case, and not an oncologist who is also occupied with caring for the patient and dealing with the clinical challenges of the advanced cancer.
But CMS Regulations Disagree
As many who work with Medicare policy know, CMS regulations require the test be ordered by the physician who is treating the patient and managing him/her. The regulation is found at 42 CFR 410.32.
- Diagnostic tests must be ordered by the physician who is treating the beneficiary, that is, the physician who furnishes a consultation or treats a beneficiary for a specific medical problem and who uses the results in the management of the beneficiary's specific medical problem.
- Tests not ordered by the physician who is treating the beneficiary are not reasonable and necessary
There are some limited abilities for a pathologist or radiologist to correct or finish a clinician's order (Benefit Policy Manual 15:80.6.4). They haven't been extended as for as, say, a Foundation Medicine or Oncotype test. OIG carefully reviewed non-treating-physician orders for genomic tests in a new fraud report regarding 81408, so it's certainly a live area of policy (here). The point is also emphasized in a current Novitas LCD for oncology genomic tests.\
CMS Legal Barrier Not Mentioned in Ellis Paper
The Ellis et al. paper talks about the improvements that would result from pathologist test ordering, but no one is quoted as being familiar with the current legal barriers that would have to be moved.
The paper includes an elaborate process map (Fig 2):
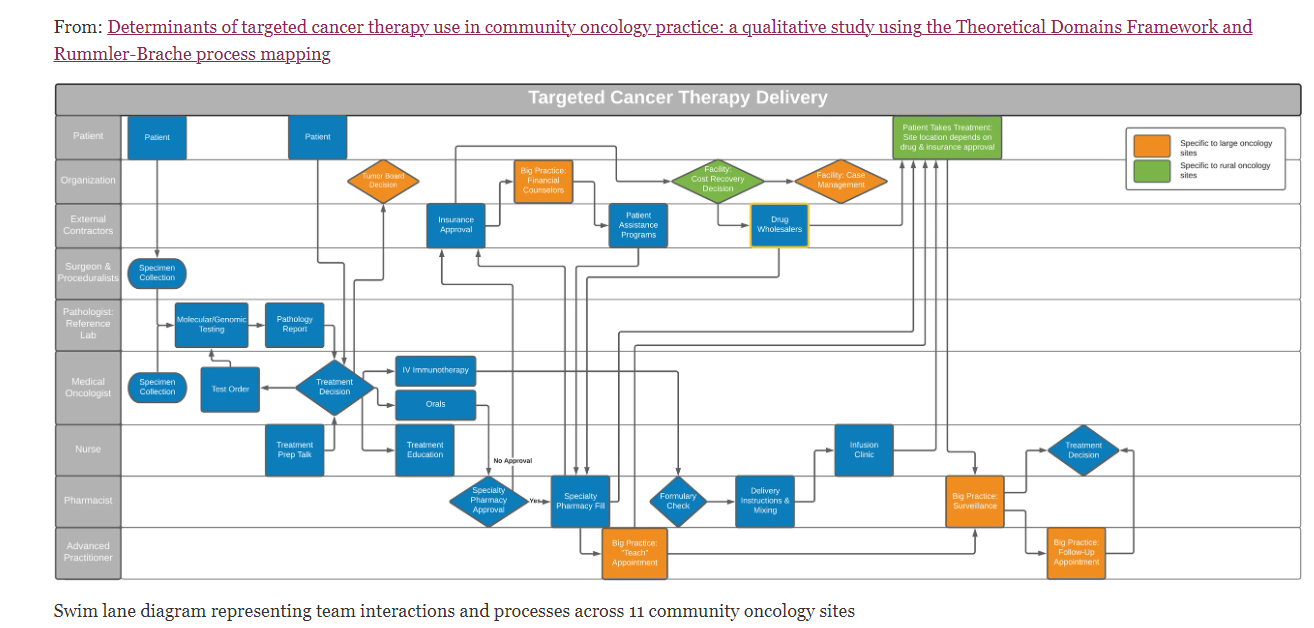 |
| click to enlarge |
They use a method called Rummler Brache process mapping from the consultancy of the same name.
___
Separate, but related, see a different Genomeweb article on cancer centers & genomic testing, by Caroline Hopkins, here.
