MolDx has released a draft non coverage LCD on "risk stratification of cutaneous squamous cell carcinoma," DL39614.
The draft LCD is posted here.
Addendum 6/16.
To shore the MOLDX program does segregate pricing from coverage, while they've issued this noncoverage LCD for the 40-gene test, they have simultaneously priced it through the Gapfill process at $3159 (0315U). (If the test does get coverage, it could be eligible for repricing as an ADLT as a covered sole source MAAA test).
_____
The coverage request, 2 pages long, seems to be dated June 1, 2020. Find it here.
There are 84 citations. Comment runs to July 15, 2023. At the bottom of the LCD, there is a link to WPS Open Meetings, referring to one on June 15.
There is a link to a draft article, DA59429. This is concise with "instructions how to receive a denial."
The Dex Registry here currently lists the Castle DxSCC test as "non covered."
See a new lengthy review of squamous cell carcinoma in NEJM, 6/15/23, here. The article notes that the BWH system is better than the usually reference-standard AJCC system. Of interest, the NEJM author Wysong is also first author of a pivotal Castle paper; his disclosure shows him as the grantee but states, no salary support. NEJM writes this regarding gene expression profiling:
Staging systems based on clinical and pathological features alone may be limited in their ability to accurately stratify all patients with cutaneous squamous-cell carcinoma. Gene expression profiling, which uses tumor biology as a prognostic factor, has been shown to be an independent predictor of metastatic risk, with a significantly improved positive predictive value, as compared with traditional staging, and similar negative predictive value, sensitivity, and specificity.[Wysong 2021] A 40-gene expression profile test has been developed and validated to stratify cases of primary cutaneous squamous-cell carcinoma into three classes (1, 2A, and 2B), with event rates for the development of metastasis at 3 years of 8.9%, 20.4%, and 60.0%, respectively.[Wysong 2021] Since this prognostic test was developed on the basis of retrospective cohorts, validation in a prospective study is needed, as well as additional data on how best to integrate gene expression profiling into clinical practice.
WALL STREET
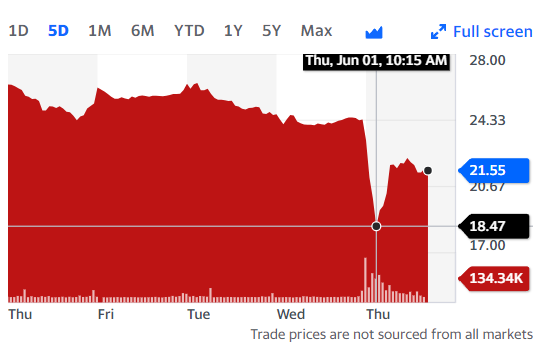
CSTL share price slipped this morning from around $24 to $19 before recovering to $21. (The one year high is $34). Against a $600M market cap, a 10% drop is around $60M value. Someone who shorted the stock at 10 am, well into the public knowledge phase, would have been well ahead at 10:30 but behind at noon.
There wasn't an immediate Castle response that I could find. In press, they recently presented data on pharmacogenetics in depression and opened a state-of-the-art lab in Pittsburgh, giving them access to both MolDx LCDs and Novitas LCDs. (Press.) 2022 had revenue around $137M, COGS at $32M, and net income around (-$67). They list 2022 R&D at $45M.
Coverage at Genomeweb here.
WHAT MOLDX SAID
Here, I quote directly from the "RATIONALE FOR DETERMINATION" in part.
Analysis of Evidence (Rationale for Determination)
- While the 40-GEP is capable of metastatic risk stratification, it is unclear how GEP-results can be consistently or accurately interpreted in the context of baseline clinicopathologic risk as part of a comprehensive risk assessment to change patient management.
- Along these lines, the literature presented to date suggests, but does not adopt, a consistent and recommended patient management strategy with regard to follow-up frequency, nodal assessment, and adjuvant therapy for Class 1, 2A, and 2B tumors according to which outcomes could be measured. Most patients receive a Class 1 or 2A result while Class 2B results are rare; therefore, it is important to clearly define the difference in management of patients with a Class 1 vs 2A result and the net benefit.
- To date, this has not been done.
- It is also important to examine the impact of changes in patient management as a result on the 40-GEP on clinical outcomes from larger real-world trials (rather than anecdotal case reports), as misclassification can cause patient harm.
- Finally, test performance has not convincingly demonstrated superiority to currently available staging tools and clinicopathologic factors in the intended use population. The main concerns are further outlined below....[continues]
_____
AI Supplement.
Separate from this blog, and only for curiosity purposes, I provide a GPT4 summary of #1 the MolDx evidence review and #2 the MolDx decision analysis - here.
These AI summaries are not endorsed by me or anyone and are presented for those curious about AI approaches.
For the evidence review the AI compresses 4300 words (omitting tables) to 450. For the analysis and conclusion, the AI condenses 2000 words to 300.
Analysis of the Analysis
Here, I am paraphrasing from remarks that MolDx made at a California meeting last winter. It's my interpretation. I would think about three classes of test or service.
1. Class 1. The new test (or service) is a standard of care. Nearly all payers and guidelines or reviews endorse it. If you have to start arguing whether it is a standard of care, you can stop, because it is not a standard of care.
2. Class 2. The new test fits into a standard of care goal or care pathway, but is better. I believe this is how MolDx gave coverage to the CareDx donor DNA test for renal transplants. We know we have to manage transplants, we know we have to detect early rejection, the test does this better than a standard, like eGFR. So it is covered. MolDx would readily admit there is a judgment factor in assessing Class 2.
3. Class 3. The test is a new test without a clear similar standard that is covered and that has an established risk benefit (unlike #2). The new test will not be covered until basic uncertainties about net risk and net benefit (real world) are resolved. MolDx would also readily admit there is a judgement factor whether a test fails the #3 standard or has enough new evidence to pass the #3 standard.
While these are my interpretations, they provide some guiderails for reading the MolDx assessment (which puts this test in group #3). Decisions over new tests are basically whether they are a better fit in Class 2 or in Class 3.