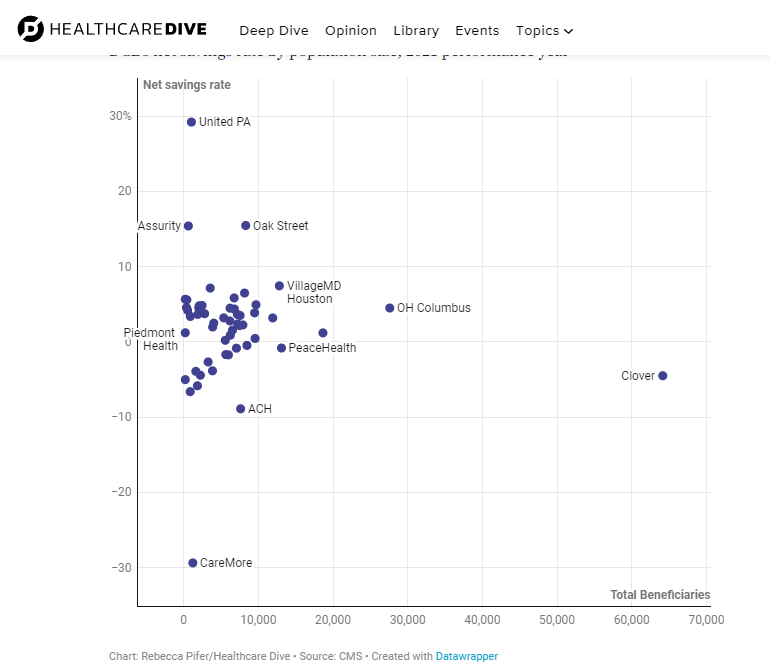
You may have heard recently about CHATGPT, a service of OPENAI.COM that is free to try.
You can ask the chatbot a question on any topic and it will respond with several sentences of text.
CNBC article here (worth reading). See a similar favorable article at Harvard Business Review here. See GPT humor here. McKinsey on ChatbotGPT here.
Landing page here; email signup required.
Before showing you CHATGPT, let me mention another open access AI service: Google BioMed Explorer. This searches PubMed, uses Google AI to go beyond the direct literal keyword search you can enter at PubMed. Worth trying.
##
CHATGPT:
When would I want genetic testing for cancer?
Genetic testing for cancer can be useful for a variety of reasons. It can help determine a person's risk of developing certain types of cancer, guide treatment decisions, and help identify clinical trials that may be appropriate for a person. Genetic testing is often recommended for people who have a family history of cancer, as it can help determine whether they may be at an increased risk for developing certain types of cancer. It can also be helpful for people who have been diagnosed with cancer, as it can provide information about the specific type of cancer they have and guide treatment decisions. In general, genetic testing for cancer is most often recommended for people who have a strong family history of cancer or who have been diagnosed with cancer and want to explore their treatment options.
##
How common are ALK gene fusions in lung cancer?
ALK gene fusions are relatively uncommon in lung cancer, occurring in about 5% of all cases. These gene fusions can cause the abnormal growth and proliferation of cancer cells in the lungs. Treatment options for lung cancer with ALK gene fusions can include targeted therapies that specifically target the ALK fusion protein.
##
Why do fraudulent providers often bill Medicare for genetic tests?
Fraudulent providers may bill Medicare for genetic tests in order to make a profit. Medicare, which is a government-run healthcare program, covers the cost of many types of medical tests and treatments for eligible individuals. This means that fraudulent providers can bill Medicare for services that were never actually provided, or for services that were not medically necessary, in order to receive payment from the government. This type of fraud is known as "Medicare fraud," and it can be a serious problem. The Centers for Medicare and Medicaid Services (CMS) works to identify and prevent fraudulent billing practices, but it can be difficult to detect and prosecute this type of fraud.
##
Sidebar: Can I make more money by becoming a criminal?
It is generally not advisable to engage in criminal activities in order to make more money. Not only is committing crimes illegal and can result in fines and imprisonment, but it can also damage your reputation and relationships with others. Furthermore, the money you make from criminal activities is likely to be unreliable and may not provide long-term financial stability. It is always better to earn money through legal and ethical means.
##
What are the differences between Medicare Part A and Medicare Part B?
Medicare is a federal health insurance program that is available to people who are 65 years of age or older, as well as some younger people with disabilities. Medicare is made up of several different parts, each of which provides coverage for different types of medical services.
Medicare Part A is hospital insurance. It covers inpatient hospital care, as well as skilled nursing facility, hospice, and home health care.
Medicare Part B is medical insurance. It covers doctor's visits, outpatient care, medical supplies, and preventive services. It also covers some home health care services.
One key difference between Medicare Part A and Part B is the way they are funded. Medicare Part A is funded through payroll taxes, while Medicare Part B is funded through monthly premiums that are paid by beneficiaries, along with a small amount of funding from general revenue.
Another difference is that Medicare Part A has no premium for most people, while Medicare Part B has a monthly premium that is determined by a person's income.
Overall, Medicare Part A and Part B are two important parts of the Medicare program that provide important coverage for a range of medical services.
##
Why do many people lack genomic testing for lung cancer?
There are a few reasons why many people may not have undergone genomic testing for lung cancer. One reason is that genomic testing can be expensive, and not everyone may be able to afford it. Additionally, not everyone may have access to a facility that offers genomic testing, particularly in more rural areas. Additionally, some people may not have been offered genomic testing by their doctor, either because it is not routinely recommended or because their specific type of lung cancer is not known to be influenced by genomic factors. Finally, some people may simply be unaware of the availability of genomic testing or the potential benefits it could offer.
##
What are AMA CPT Category III Codes?
AMA Category III codes are codes used by the American Medical Association (AMA) to identify new and emerging technologies, procedures, and services that are not yet widely used, but are showing promise and may become more commonly used in the future. These codes are part of the AMA's Current Procedural Terminology (CPT) code set, which is used to report medical procedures and services to insurance companies and other healthcare payers. Category III codes are intended to provide a way for healthcare providers to be reimbursed for these new and emerging services, which may not have a corresponding code in the main CPT code set.
##
What is Chromothripsis?
Chromothripsis is a rare and poorly understood phenomenon in which a single cell undergoes a massive and catastrophic rearrangement of its chromosomes. This can result in the cell acquiring many new genetic mutations, which can lead to the development of cancer or other diseases. Chromothripsis is thought to occur when a cell experiences a severe physical trauma, such as exposure to high levels of radiation or a physical blow to the cell. The exact mechanisms underlying chromothripsis are not well understood, and further research is needed to better understand this phenomenon and its potential implications for human health.
##
Describe the importance of oncogenes in cancer in the style of the King James Bible.
Behold, the oncogenes are a powerful force in the realm of cancer. For when these genes, which are present in all living cells, are changed or corrupted, they can unleash a tide of uncontrolled cell growth and division. This can give rise to malignant tumors, which, if not checked, can spread throughout the body and bring misery and death. It is therefore of great import that we study the oncogenes, and use this knowledge to develop ways to combat this deadly scourge.
Describe the role of oncogenes in cancer, in the style of a kindergarten teacher.
Oncogenes are special genes that help cells grow and divide. Normally, these genes keep our cells healthy and make sure they grow at the right time and in the right way. But sometimes, oncogenes can get damaged or changed, and this can cause them to start making too many copies of themselves. When this happens, it can cause cells to grow out of control and form a mass of tissue called a tumor. Tumors can be either benign, which means they are not cancerous and won't spread, or malignant, which means they are cancerous and can spread to other parts of the body. Oncogenes play a role in cancer because they can help cause cells to become malignant and form tumors.
Finally, let's try asking the same question, but with opposite expectations:
Why is it a bad idea to have testing for pharmacogenetics?
While pharmacogenetic testing can provide valuable information about a person's genetic makeup and how it may affect their response to certain medications, it is not always a good idea to undergo this type of testing. This is because pharmacogenetic testing can sometimes produce false positive or false negative results, which can lead to incorrect or misleading information about a person's ability to tolerate a particular medication. Additionally, pharmacogenetic testing can be expensive, and it is not always covered by insurance. Furthermore, there are ethical concerns about the use of pharmacogenetic testing, including the potential for discrimination based on genetic information. For these reasons, it is important to carefully weigh the potential benefits and drawbacks of pharmacogenetic testing before deciding whether to undergo this type of testing.
Why is it a good idea to have testing for pharmacogenetics?
While there are potential drawbacks to pharmacogenetic testing, there are also several reasons why this type of testing can be beneficial. For example, pharmacogenetic testing can provide valuable information about how a person's genetics may affect their response to certain medications, which can help doctors prescribe the most appropriate and effective treatment for their patients. This can reduce the risk of adverse drug reactions and improve the overall effectiveness of treatment. Additionally, pharmacogenetic testing can help identify individuals who may need to be carefully monitored or who may require alternative medications to avoid potentially dangerous side effects. Furthermore, pharmacogenetic testing can be particularly useful for people who are taking multiple medications or who have complex medical conditions, as it can help doctors tailor treatment to the individual's unique genetic profile.
##
Will genetic testing make it possible for me to fly to the moon?
No, genetic testing will not make it possible for you to fly to the moon. Genetic testing is a medical procedure that involves analyzing a person's DNA to identify genetic variations or mutations that may be associated with certain traits or health conditions. This type of testing has no bearing on a person's ability to fly to the moon or engage in other physical activities. Flying to the moon requires advanced technology and extensive training, and is only possible for a select few individuals who are chosen to be astronauts by space agencies such as NASA.
How I heard about ChatGPT here.