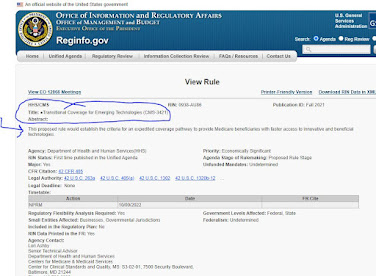
On January 11, 2022, CMS released a proposed decision on the class of present and future anti-amyloid Alzheimer drugs, allowing them to be covered only in a randomized clinical trial (entry point here).
This is called "CED" or "Coverage with Evidence Development." Payment for services under CED, and the rules for when it is OK or not OK, are predicated on an obscure phrase in the Medicare statute that has become controversial from time to time.
In fact, the Trump administration issued an order in January 2021 that CED, as currently used, was not legal under the statute. This blog revisits some of the links.
- See the five minute YouTube version of this article, here.
____
Typical Medicare Statute: Benefit at 1861, Restriction at 1862
Early in the Medicare statute, there is a phrase that says Medicare is a program for medical and other health services. Then, further along, at SSA 1861, we find definitions (such as "physician") and this section includes a definition of what Congress means by "Medical and Other Health Services," placed at SSA 1861(s).
The term means "physician services" (see definition of a physician!), "services incident to a physician," "diagnostic tests," and so on. This goes on for 2000 words, and while some benefits are 3 words and some are 50 words, let's say the average is 15 words - that's 130 benefits, more or less. For example, "physician services" are a two-word benefit, while an "oral drug used as anti cancer drug" is a 65 word benefit.
OK, that defines benefits.
What are the restrictions? These are found at SSA 1862. Most famously, see 1862(a)(1)(A), medical may not pay for services that are not reasonable and necessary to diagnose or treat disease. So 1861 tells us that physician services, and diagnostic tests, and oral anti-cancer drugs (among many things) are Medicare program benefits, while 1862 tells us that payment is impossible if they are not reasonable and necessary.
CED Justification Actually Found at 1862 - As a Restriction to Medicare
The justification to CED is actually found, not at 1861 (where clinical studies of AHRQ might have become a defined benefit) but at 1862 (where services not reasonable and necessary for AHRQ studies, are NOT a benefit.)
At 1862, we find the odd phrasing:
- No payment may be made under Part A or B for any expenses for items and services {necessary for treatment of illness} {except for...}
- (E) in the case of research conducted pursuant to SSA 1142, which not reasonable and necessary to carry out the purposes of that section.
SSA 1842 is AHRQ law.
So when CMS says CED is justified by SSA 1862(a)(1)(E), what they are pointing to is a law that CMS cannot pay for services not fulfilling the goals of SSA 1142. It never says that CMS "can" pay for services fulfilling the goals of SSA 1142.
So we can summarize how Section 1861 and 1862 work in this table:
 |
| Click to enlarge |
But Wait, There's More: Confusing Adjacent Uses of "Reasonable and Necessary" Phrase
Notice that the term "reasonable and necessary" is being used in completely different ways. In 1862(a)(1)(A), "reasonable and necessary" is in regard to human medical care for patients with diseases. OK, we know what that means. In 1862(a)(1)(E), "reasonable and necessary" is used in regard to "purposes of AHRQ research" which is a completely different domain or concept.
While SSA 1142 is long, 1700 words, and the word "support" occurs 11 times, the top line purpose of AHRQ in this section of law is to "conduct and support research with respect to outcomes, effectiveness, and appropriateness of health care procedures."
What CMS Thinks (2014)
CMS, as an agency, believes it has broad authority through National Coverage Determinations, to issue coverage restricted to clinical trials, or registries, or other research pathways, as it sees fit. CMS last elaborated on the legal status and implementation policy for CED in a long web article on the CMS website in 2014 - here.
What General Counsel at HHS Thought in January 2021
There have been multiple cycles of legal debates regarding what the CED law (e.g. the phrasing at 1861(a)(1)(E) pointing to SSA 1142) means. Note that this section is phrased in the negative; CMS an't cover services not reasonable and necessary to treat disease, and can't cover services not necessary to support SSA 1142 aka AHRQ.
January 2021. In January 2021, the outgoing Trump administration posted a legal Advisory Opinion of General Counsel of HHS, at that time led by attorney and HHS Secretary Alex Azar. While later deleted online by the Biden administration, a cloud copy is here. This memo provides some behind-the-scenes insights. For example, it says in the agency file (internal file) for each NCD with CED, there is an email or letter from AHRQ saying it "supports" the NCD.
The key point is: Then-HHS General Counsel Robert Charrow opined that such a "note to file" is insufficient to meet the standards of "support" in its reasonable meaning in place in the legislation.
(I would put it this way. If we hear that, "NIH supports the Johns Hopkins Cancer Institute," you wouldn't expect that to mean solely that someone at NIH sent an email to Johns Hopkins, "Fine cancer center - keep it up" and thus "supporting" the cancer center.)
Lilly's Arguments Against CED - February 2022
In comments regarding the Aduhelm NCD in February 2022, Lilly provides several paragraphs of arguments on why CED is not legal, at page 5-6-7 of this comment document (here).
Understanding New NCDs that Point to 1861(a)(1)(E)
This provides some background why all the CED NCDs, such as the Amyloid Drug NCD just proposed, carefully refer to 1861(a)(1)(E), and carefully cite AHRQ as an cooperating agency.
The first point of this article is that CED is so debated, and requires pages of justification by CMS, because the statutory background is so weird and implicit. Look: CMS never write a 20-page memo justifying if it can provide hospice benefits, because statute at 1861(dd) point-blank creates hospice benefits. No such section at 1861 affirmatively creates AHRQ support.
My second contribution would be to point out that judging whether a service - like this pill here for John with this disease - is "reasonable" and is "necessary" to treat a disease is very different than asking whether something like "a clinical trial" is "reasonable" and "necessary" to support a goal of an abstract entity like an agency. (Or at least, what an agency "wants" and "needs" and how it has a "goal" or "purpose" is abstract, in a way John on a certain day and in a certain medical office, and his cancer, and his pill are not.)
____
Other Notes
Book. Twenty years ago, in 2000, National Academies of Science released a book on "Extending Medicare Reimbursement in Clinical Trials." It's still online as a free PDF here.
Little-Known Payment Law. MIPPA 2008 law added a clause to Medicare payment policy, at SSA 1833(w), allowing Medicare to alter payment rules in the context of clinical trials. This has no implementing regulations or policies, and I'm not sure if it's ever been used (as a self implementing regulation) but the idea would be to adjust or change copays and payments to preservice blinding (masking) in RCTs that have double blinding.
"Reasonable and Necessary." AT 1862, the phrase is used first in regard to health care and immediately later, in regard to goals of AHRQ. Yet there must be a sea change in meaning between one phrase and the other of the identical twin usage. For example, CMS defines "reasonable and necessary" as "not investigational," but everyone would define an RCT as "investigational." CMS would never define a placebo as "necessary" to treat disease, but a placebo might be "necessary" toward a trial that is a research goal of AHRQ.
CMS phrase, "Trials supported by NIH." Charrow wanted to define "support" narrowly and CMS defines "support" broadly (e.g. AHRQ note to file stating "support.") This broad definition at CMS becomes very helpful when we turn to CMS supporting (in CED) trials "supported" by NIH, since NIH wouldn't need to fund the trial, or mostly fund it, but only "support" the trial. Charrow wanted to define "support" narrowly and CMS defines "support" broadly (e.g. AQRQ note to file stating "support.") This broad definition is helpful when we turn to CMS paying for (in CED) trials "supported" by NIH, since NIH wouldn't need to fund the trial, or to mostly fund it, but only to "support" the trial.
What I think it all meant. Clearly, Congress meant "something" by 1862(a)(1)(E). I think they intended that AHRQ would plan trials that included routine clinical costs, experimental clinical costs, and administrative costs (which can be large). CMS would pay routine clinical costs anyway. 1861+1862 allows CMS to pay necessary clinical costs that were beyond routine clinical costs. AHRQ would still pay for administrative costs or other costs (e.g. AHRQ plans and "supports" the trial.) As it is, CMS has pretty much unfettered CED at scale. A CED trial with 10,000 patients and a $30,000 drug would involve $300M dollars (or $150M if discounted for half placebo). An NIH trial at that scale would never exist but other large NIH trials would have enormous amount of vetting before NIH spent even $10M or $20M.
1862(a)(1)(E) was Drafted to be Confusing. The more times I re read 1862(a), the more the piled-up clauses and "except for's" and double negatives are confusing. It would be simpler if 1861(s) had a written benefit for support of AHRQ trials, and then regulations and rulemaking as to what that means.
CMS Coverage in Clinical Trials - Dates to 1980. See a fascinating 2017 paper by Roger Evans, who was involved in 1980 decisions about heart transplant policy. I wrote about the era, informally, in a 2015 blog.
SCOTUS: Legislators Do Not Hide Major Principles. I argue here that the CED law is, at best, murky. There are long CMS arguments about if, when, or how it can do CED at all. In contrast, there are no long murky arguments if CMS can offer hospice benefits, you just point at 1861(dd), or physician benefits (1861(s)(1)), or diagnostics (1861(s)(3)). The current SCOTUS case about CMS requiring health system personnel vaccinations notes "We presume Congress does not hide fundamental details of a regulatory scheme in vague or ancillary provisions," Whitman v American Trucking, SCOTUS, 2001.
__
Charrow also wrote a book, Law in the Laboratory, 2010, here.