MolDx has issued coverage for the Oncocyte VitaGraft renal rejection test, per a press release here and brief coverage at 360DX here,
The relevant organ rejection LCD is a broad, or foundational LCD, that allows the potential for future coverage of molecular testing for graft rejection in any organ. Actual coverage issued has included tests using donor DNA detection, from CareDx and Natera. There have been some kerfuffles about the LCD, its textual analysis, and revisions.
Price Pending
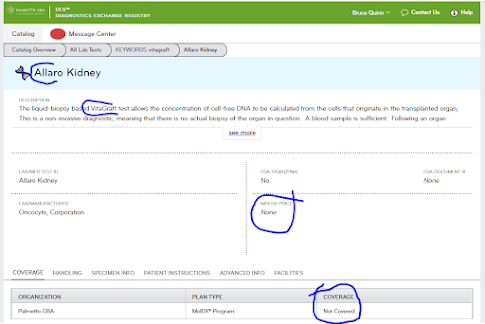
As of August 29, the Palmetto "DEX" database did not yet list the Vitagraft test as covered, and, it had no Medicare price list. Updates will likely appear online over a week or two.
The DEX database refers to the "Allaro" Kidney test, whose description then calls itself the VitaGraft test [?]. One year share price is down from $18 to $3.50. Market cap is $30M. For 2022, "full-year 2022 revenues were $5.6 million, down 27 percent from the prior year. Net loss for the year was $72.9 million." The company has bounced in and out of Nasdaq compliance. The company has immuno-oncology test assets that are not yet covered by MolDx (Determa IO).
ddPCR and MolDx
Although examples are still not too common, digital PCR has potential applications in graft rejection and minimal recurrent disease detection in oncology.
 |
| click to enlarge |