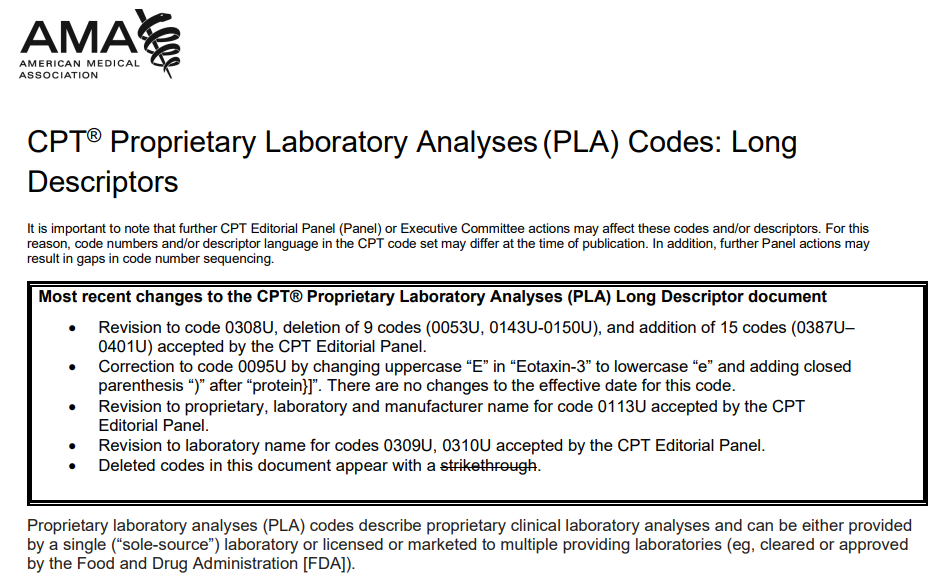
AMA publishes the results of first quarter PLA applications. The publication includes October and January PLA codes, which will also be part of this summer's pricing meeting.
###
The AMA CPT accepts "PLA" proprietary lab code applications on a quarterly basis (e.g., January 1, April 1) and announces new decisions every quarter (e.g., April 1, July 1).
On March 31, the AMA posted decisions regarding PLA applications submitted in early January, which were voted on during the February AMA CPT meeting. In addition to several code deletions, new codes range from 0387U to 0401U, totaling 15 codes.
This publication is particularly useful as it compiles all the codes submitted too late for inclusion in the AMA CPT 2023 fall edition. Essentially, these codes will be discussed in this summer's CMS pricing meetings, as they missed the previous summer's session. The only remaining PLA codes to be added are those that will be submitted on April 6. The AMA is expected to finalize this batch of codes in early May, which should make them eligible for consideration during the CMS lab pricing meetings in June and July.
Here's the link to the new PLA codes:
https://www.ama-assn.org/system/files/cpt-pla-codes-long.pdf