Key point: You can look at labs named in public website DOJ documents, and in a few moments, see exactly what codes and claims they were paid for in 2020 by Medicare Part B.
____
What Are Tier 2 Codes?
Back around 2013, AMA CPT brought out a new coding system for lab tests. Tier 1 codes were named codes (e.g. BRCA or EGFR) numbered 811xx to 813xx. Tier 2 codes were 8 "buckets" or complexity levels, any one of which might refer to dozens or hundreds of possible genetic procedures (e.g. procedures that measure 2 or 3 exons or procedures that measure 4 to 9 exons). These were numbered 81401-81408.
2017: Low Volumes of 814XX Payments
These codes were not initially priced by CMS, and payments as late as 2017 were only around $72M, of which $9M or 13% of payments were for 81408, the highest code which pointed to full sequencing of rare genes. (They were rare genes, hence, that's why they weren't Tier 1 codes).
In 2018, with the first PAMA pricing cycle, all the Tier 2 codes were suddenly priced, and priced as high as $2000 for 81408.
The Tier 2 Industry Grew Rapidly 2018-2020
The Tier 2 coding industry grew rapidly, based on Medicare Part B data that is public. In 2018, payments rose to $190M (of which $120M, or 65%, for 81408). In 2019, payments rose to $381M, of which, $284M or 74% for 81408. In 2020, payments slipped a little to $358M, of which $206M or 58% for 81408. CMS will release 2021 data in Fall 2022.
In July, Medicare released detailed Part B payment data for every lab (or doctor) and CPT code, for CY2020. You can get all the data from CMS via easy to use public web resources here.
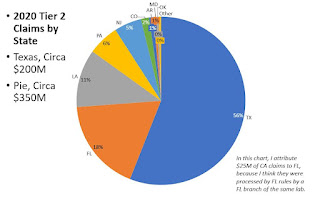
DISTRIBUTION BY STATE: DON'T MESS WITH TEXAS
Relative to all US genetic payments, the pattern for Tier 2 payments is unusually, with 56% of payments going to 1 state, Texas.
Together, TX, LA, and FL get 78% of payments, and PA/NJ add 11%. These are all Novitas (or FCSO) policy states.
The table below shows a lab in California, a MolDx state, getting $26M for Tier 2 codes, but I understand from other research that MOLDX generally doesn't pay for Tier 2 codes, or very little, and so this CA listing may be an anomaly related to an NPI or Tax ID issue. If we stipulate that the CA listing is some kind of anomaly (doesn't reflect Noridian claims), then all the Tier 2 payments, practically, were in Novitas and FCSO states.
DISTRIBUTIONS OF LABS FOR EACH PLA CODE
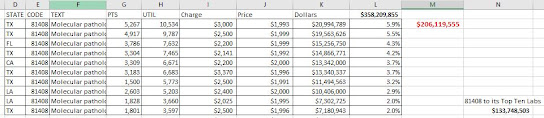
You can also take the largest PLA code, 81408, and see how its 2020 utilization was distributed among individual Medicare labs. (The original CMS data, linked above, includes lab names, street addresses, etc).
About 100 labs had at least a few payments for 81408, but the top two labs had about 10,000 patients each for about $20M each. Of the 100 labs billing 81408, the top ten garnered $134M of the $206M available in 2020. (One lab had about 4 claims of code 81408 per patient).
DOJ Telemedicine/Genetics Documents
I leave as an exercise for the reader. The DOJ has a long webpage of cases it is pursuing related to telemedicine orders for genetic tests (in some cases DME equipment). Here:
https://www.justice.gov/criminal-fraud/telemedicine-court-documents
If you look through the DOJ documents for named labs, and then, refer back to the public CMS claims database I've been talking about, you can see in a few seconds the paid claims billing pattern for each lab that is named and cited by the DOJ. It's public, and literally at one's fingertips.