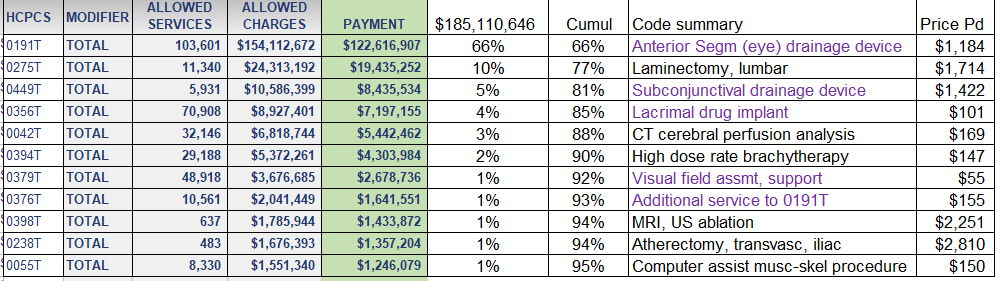
There are a few good papers on micro-costing for next generation sequencing and other forms of genomics, but not a lot. And when they are produced, they're often vague on the critical role of overhead, or ignore that variable completely.
Here's a comprehensive look at micro-costing for circulating tumor DNA testing, November 2022, from Kramer et al, a Dutch group. The authors take a comprehensive approach, including "personnel, materials, equipment, overhead, and failures." Alternative policies and protocols give outputs that range from $199 to $9124 per sample (most scenarios $300-1000, all-in total costs and overhead).
Note, however, that the models are academic and don't account for actually running a business or running clinical trials for products, or handling prior auth, dealing with diverse payors, or regulatory approvals. They specifically remark that some important costs were left out, even with this elaborate model. Find the article here and open-access, with supplements including the Excel model:
https://www.jmdjournal.org/article/S1525-1578(22)00309-9/fulltext
There's an old joke that you ask an economist or an accountant how much is 2+2, and he asks, 'How much do you want it to be?" Behind that, there are different accounting systems for different purposes. I recall from MBA school, there are at least three entirely different accounting systems to be aware of, financial accounting (generally accepted accounting principles, GAAP); tax accounting (following tax law); and activity-based accounting. Other types of accounting are designed for investors, e.g. return on equity. More recently, see challenges in the accounting for entities that are primarily intangible assets (see Capitalism without Capital, by Haskel & Westlake). Unfortunately, in "applied economics," even basic accounting terms can be badly unused, often with blissful ignorance of the mistakes. ("I didn't go to four years of medical school [graduate school] [residency] to use common accounting terms correctly.")
Kramer et al. have one of the most intellectually interesting approaches I've seen. Besides a diverse range of inputs and overhead, plus accounting for batch size or total samples per year, they provide supplemental tables that cover 5 different methodologies:
- Digital PCR,
- PCR (model 1),
- PCR (model 2),
- Mass spec,
- NGS.