MolDx has a new hereditary cancer testing LCD this summer, which is heavily dependent on two supporting documents, a billing and coding article, AND, a technical assessment worksheet in Excel.
Longstanding LCDs like L36163 (BRCA testing) and L36370 for Lynch syndrome have been retired. (*)
In their place is a composite LCD for all hereditary cancer syndromes, L38972. This basically states that hereditary cancer panels are covered if the patient has a (personal) cancer diagnosis, a "clinical indication" for germline testing, a "risk factor" for inherited cancer, the test has completed a MolDx TA, and in particular:
- The test performed includes at least the minimum genetic content (genes or genetic variants) with definitive or well-established guidelines-based evidence required for clinical decision making for its intended use that can be reasonably detected by the test.
- Because these genes and variants will change as the literature and drug indications evolve, they are listed separately in associated documents, such as the MolDX® TA forms.
The LCD conforms to requirements in NCD 90.2 for NGS testing in cancer patients. (However, the tests are not required to be NGS tests, as occurs in NCD 90.2).
- TIP: NCD 90.2 defines what is a CMS "clinical indication" and "risk factor" which I can never remember without looking it up (more here). A risk factor is "not a clinical condition" (e.g. age is a risk factor, and so is family history) but a clinical indication is something "clinical" like a medical sign, symptom, or test result. The LCD requires a personal history of cancer, AND a clinical indication (not listed) AND a clinical risk factor (not listed); but the NCD directly defines a personal history of cancer as already being a clinical indication (previous link).
The LCD is then dependent on a billing article, A58679. This MolDx billing article generally blocks the use of 81162, the standard two-gene BRCA code, because there is no cancer where this is the only gene recommended for testing. Since "medically necessary use of BRCA" only occurs in panels, MolDx requires it to be billed in panels (either a CPT panel like 81432 or a MolDx local panel under code 81479.)
But wait, there's more: MolDx TA Article Associates Genes and Cancers
But wait, there's more. Every panel covered under LCD L38972 must be approved through a MolDx tech assessment, which is Form NGS PF 006 V5. Find it on the MolDx technical assessment page here. (While you're at it, don't forget to carefully study the FAQs as well, here.)
Form NGS PF 006 V5 has been updated effective August 31, 2022.
Comparing V4 and V5
I've put an archival August 26 version of V4 in the cloud here.
The original version had one tab, beginning with the Z code for the test, and the indications for use (e.g. "breast cancer.") Note that guidelines tend to view these hereditary tests categorically, like "HBOC" (hereditary breast and ovarian cancer) and "Lynch syndrome." However, Medicare requires the patient to have a particular personal cancer, so "breast cancer" is the entry point, not 'HBOC" or "Lynch." MolDx then asks several preanalytical questions (#4), analytical questions in general (#5), and post analytical questions (#6, eg "Who signs out these results?") Question #7 asks for sample types, and the largest question was Question #8, detailed benchtop validation data in clinical (or non clinical) samples.
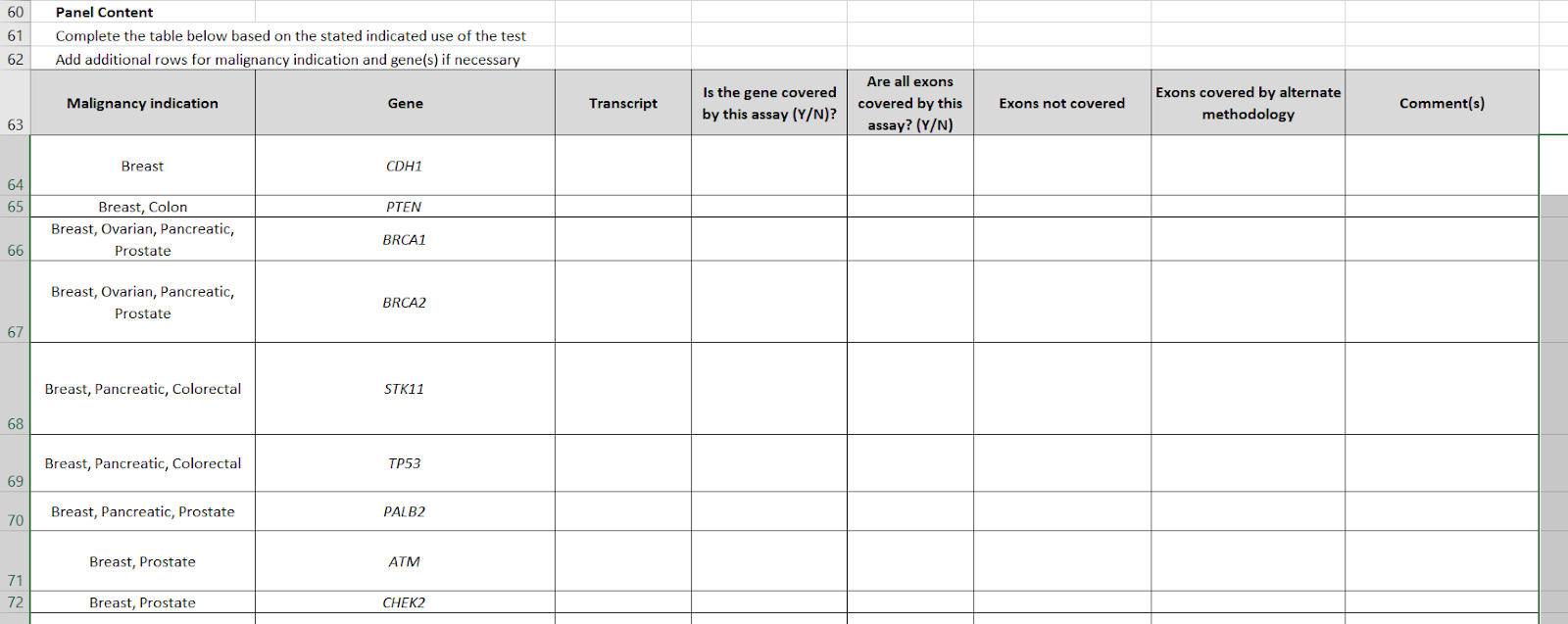
Then, a key table was #9, required genes. This listed 24 genes and which cancers each one was required for.
OK, now update to V5.
V5 is divided into three tabs.
The first tab is "test information" and requires an attestation that the information is accurate to the best of the lab director's knowledge and was performed in the CLIA lab associated with the Z code, not somewhere else. (There were some labs cited in Operation Double Helix and other DOJ operations that primarily referred genomic tests out to some other lab, said Reuters.)
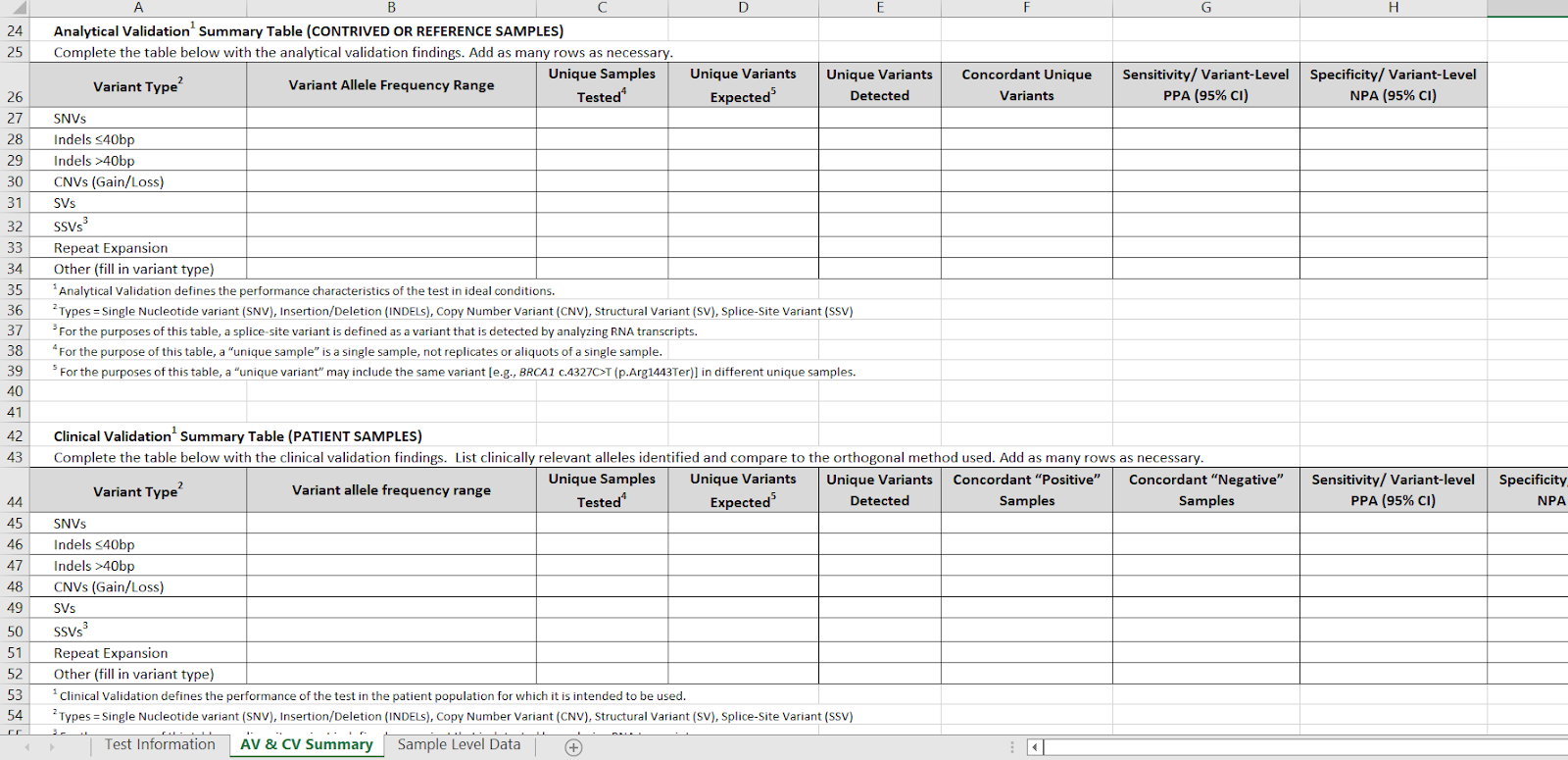
Tab 2 has separate detailed tables for analytical validation on contrived or reference samples (e.g. Coriel) and clinical PATIENT validations. These two tables are now placed adjacent to one another for easier comparison.
The table for gene-cancer associations has been inverted. Whereas V4 was a list of genes, and a column with the names of cancers, now we have "malignancy indication" as the first column, which may be multiple (e.g. STK11 is "breast, pancreatic, colorectal") and a gene list. The gene list has been raised from #24 to #35. In particular, conditions like tuberous sclerosis and neuroendocrine cancers, have been added.
There is now a third tab, which is "Sample level data." This table has 7 columns: Sample identifier, Cancer type, sample type, Variant class, Gene (BRCA), Variant (C4327C>T), and variant class ("Pathogenic.")
___
___
(*)
MolDx LCDs and articles have different reference numbers depending on the jurisdiction; I have used Noridian reference numbers here.