See a follow up in September 2022 here.
_____
I'm going to jump to the "master class" level with this one, with just a brief preface. Medicare bundles ("packages") most lab tests performed in the hospital outpatient setting (include hospital outpatient surgery and hospital outpatient visits and hospital based ER's), unless they meet an exception, primarily, human DNA-RNA tests.
CMS publishes an outpatient claims-processing table called "Appendix B" where payable tests are "A" and packaged tests are "Q4."
See a screen shot below.
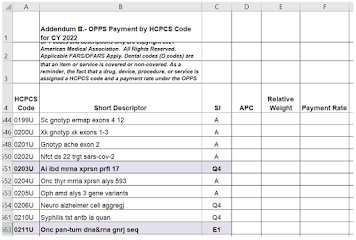 |
| click to enlarge |
Most of the Status Indicators (SI) make sense to me, but not all.
0199U-0201U are "A", payable, as human DNA RNA tests. 0202U is also payable, because it contains a COVID report (special rule). 0204U, 0205U are "A" as human DNA RNA tests.
But as an expert, I can't always figure out what CMS is thinking.
0203U is an RNA expression test for inflammatory bowel disease (PredictSURE) from whole blood, and I'm pretty sure it's WBC RNA expression, so it should be "A" rather than the published Q4 nonpayable.
And 0211U is the Caris comprehensive tumor genomics test, and it's the rare status E1 which means not covered by any outpatient benefit category. I don't know why this isn't "A," the similar Foundation Medicine test 0037U is "A" and so are many other complex tumor tests with PLA codes.
__
See status indicators table here. Find updates to Appendix B OPPS, here. CMS publishes a quarterly update classifying new lab codes with letter designators, find one recent example here.