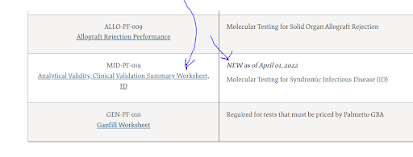
Now on April 1, 2022, we have the last major part of this policy event. MolDx has published a detailed template, in Excel format, for infectious disease panel testing, with performance and quality criteria that must be meet. Find it on the link, look under the section "MID-PF-019, Analytical & Clinical Validation Summary Worksheet for ID."
For me the Excel link is here, but that could change over time.
Per email, MolDx confirmed to me the panel TA is required for 2 or more pathogens. It is *not* required for strains of 1 pathogen (e.g. HSV-1 and HSV2- does not trigger the "panel" TA).
Not that MolDx defines panel as 2 or more, including 2-5, but AMA CPT has several panel codes for the terminology "3-5 targets, 6-11 targets, 11-25 targets," so the MolDx species terminology and the AMA CPT wording might not line up. I'll leave the details to MolDx and ID experts.
requests, which also speeds the review time at MolDx itself.
Sections include:
- General Test Information
- Accuracy EP-9A2
- I believe EP9A2 is a reference to a CLSI lab guideline document
- Analytical Sensitivity EP17A2
- Precision (Qualitative, Quantitative) EP05A2, EP12A2
- Minimum Input Quality MM09A2
- Quality Controls and Management
- Five extra questions, e.g. "Is antimicrobial sensitivity testing performed (AST?)"
- List CPT/PLA code (or suggest crosswalk if N.A.)