some of the workload.
CHAT GPT Weighs In
I "interviewed" ChatGPT4 about the materials. I'm printing the answers below, because they match my understanding of the facts.
Summary of the Six Parts of the TCET in Federal Register:
The 11,000 word document was given to ChatGPT in 2000 word parts.
Part 1: This part introduces the Centers for Medicare & Medicaid Services (CMS) and the purpose of the document. It explains the need for a new pathway called the "Transitional Coverage for Emerging Technologies" (TCET) pathway, which aims to provide Medicare coverage for breakthrough medical devices that have the potential to significantly improve patient outcomes. The key objective is to expedite coverage decisions for these devices while generating additional evidence to support long-term coverage determinations.
Part 2: Part 2 describes the criteria for a medical device to be eligible for the TCET pathway. It outlines the characteristics that define a breakthrough device, including FDA Breakthrough Device Designation, providing evidence of substantial clinical improvement over existing technologies, and addressing an unmet medical need. The document also emphasizes the importance of the manufacturer's commitment to collecting additional evidence through post-market studies.
Part 3: This section explains the process of nomination for the TCET pathway. It describes how manufacturers can submit nominations and the required information, including device details, evidence supporting its breakthrough status, and a comprehensive plan for evidence development. The document emphasizes the importance of clear and concise communication between the manufacturer and CMS throughout the nomination process.
Part 4: Part 4 focuses on the steps involved after a nomination is submitted. It highlights the need for manufacturers to promptly submit nominations to facilitate alignment among CMS benefit category determination, coverage, coding, and payment considerations. It provides guidance on how manufacturers can submit nominations and the information required to support their nomination. It also encourages manufacturers to provide additional specific information to facilitate benefit category and coding determinations.
Part 5: This part delves into the review and coverage process under the TCET pathway. It outlines the timeline for reviewing nominations, conducting evidence previews, and developing evidence development plans (EDPs). It emphasizes the importance of stakeholder input, particularly from specialty societies and patient advocacy organizations, in shaping coverage policies. The document also discusses the duration of transitional coverage under the TCET pathway and the transition to post-TCET coverage.
Part 6: The final part of the document highlights the roles of different participants in the TCET pathway, including manufacturers, CMS, FDA, and the Agency for Healthcare Research and Quality (AHRQ). It mentions the availability of other expedited coverage mechanisms like Parallel Review and emphasizes the need to prioritize innovative medical devices with the potential to benefit Medicare beneficiaries. The document also mentions the exemption of information collection requirements under the Paperwork Reduction Act and addresses the response to public comments.
Overall, these six parts provide a comprehensive overview of the TCET pathway, its purpose, eligibility criteria, nomination process, review and coverage process, stakeholder involvement, and the roles of various entities involved.
How does the new process handle breakthrough devices?
ChatGPT
Yes, the new process outlined in the document specifically addresses the handling of breakthrough devices. It is a program for such devices.
Breakthrough devices are the focus of the Transitional Coverage for Emerging Technologies (TCET) pathway. The document states that only breakthrough devices, as designated by the FDA, are eligible for consideration under the TCET pathway.
The criteria for a breakthrough device are described. FDA follows regulations to determine there is evidence demonstrating substantial clinical improvement over existing technologies [this might be phase 1 or phase 2 evidence], and addressing an unmet medical need. Devices meeting these criteria are considered breakthrough devices and are eligible for nomination under the TCET pathway.
CMS explains the process of nomination for the TCET pathway, emphasizing that manufacturers can submit nominations for breakthrough devices. The manufacturers are required to provide comprehensive information about the device, evidence supporting its breakthrough status, and a detailed plan for evidence development.
Throughout the document, there is an emphasis on the importance of manufacturers committing to collecting additional evidence through post-market studies. The TCET pathway aims to provide expedited coverage for breakthrough devices while generating additional evidence to support long-term coverage determinations. This highlights the recognition of the unique potential of breakthrough devices to significantly improve patient outcomes and the need to streamline their coverage and evidence development process.
CMS has previously had a process called "PARALLEL REVIEW" where the company, FDA, and CMS met in advance of a CMS policy decision, and a CMS policy decision was crafted for quick release timed with the FDA approval. That was touted as a big deal 10 years ago, but have almost never been used. How is this new program different?
How do we know it isn't the familiar unsuccessful Parallel Review program with a new hat?
ChatGPT
The new TCET pathway mentioned in the document shares some similarities with the previous Parallel Review program, but also introduces significant differences. Here are the key distinctions between the two programs:
Focus: The Parallel Review program focused on the concurrent review of both FDA approval and CMS coverage determination, aiming for synchronized decision-making. In contrast, the TCET pathway primarily focuses on rapidly defining post-market evidence development for breakthrough devices, to facilitate coverage under the Medicare program.
Timing: The Parallel Review program involved simultaneous review and decision-making. The TCET pathway allows for earlier engagement between CMS and manufacturers, beginning with the nomination stage before FDA market authorization. But much of the TCET also occurs in the months immediately following FDA approval. The TCET pathway prioritizes upfront planning and then ongoing collaboration for evidence development, rather than a simultaneous decision-making process and only before FDA approval.
Evidence Development: The TCET pathway places a significant emphasis on evidence development during the post-market phase. It requires manufacturers to submit a detailed Evidence Development Plan (EDP) outlining how they will collect additional evidence to address any existing evidence gaps. The EDP must be approved by CMS and the Agency for Healthcare Research and Quality (AHRQ). The TCET pathway recognizes the need for generating real-world evidence and addressing uncertainties surrounding breakthrough devices.
Coverage Timing: The TCET pathway acknowledges the time-sensitive nature of coverage decisions and aims to provide coverage under Medicare within 6 months after FDA market authorization. This timeline sets a goal for faster coverage compared to the traditional NCD process.
While the previous Parallel Review program had limited utilization, the TCET pathway introduces a new approach that aligns with the need for evidence development and timely coverage determination for breakthrough devices. The TCET pathway's primary focus on evidence generation, early engagement, and post-market studies distinguishes it from the earlier Parallel Review program.
How will they handle bandwidth under TCET?
The CMS NCD process is known for being slow and having very little bandwidth per year.
ChatGPT
The document acknowledges the resource constraints and limited bandwidth of CMS in handling coverage determinations. To address this concern, the TCET pathway aims to prioritize innovative medical devices that have the potential to benefit a significant number of individuals with Medicare. The document indicates that CMS anticipates accepting up to five TCET candidates annually due to resource constraints, but those five will be devices impacting the most Medicare beneficiaries.
CMS plans that some workload can be outsourced to contractors.
By focusing on breakthrough devices and implementing a streamlined evidence development process, the TCET pathway intends to expedite coverage decisions. The goal is to finalize the Evidence Development Plan (EDP) approximately 90 business days after FDA market authorization, and the overall timeline for providing coverage under the TCET pathway is intended to be shorter than the traditional National Coverage Determination (NCD) process.
While the document does not provide specific details on how CMS will manage the bandwidth, it acknowledges the need for efficiency and timely decision-making. By prioritizing candidates with the potential for broader impact and leveraging the EDP for evidence generation, the TCET pathway aims to optimize resources and streamline the coverage process for breakthrough devices while choosing devices that benefit the most patients.
The program seems to hinge on "CED" which is Coverage with Evidence Development. CMS pays for Product X, but only in a clinical trial defined and judged by CMS.
Some academic papers (see one at ZEITLER) have said that CED clinical trials are often unfinished, never start, fade away, and have rarely been known as impactful respected clinical trials with important results. Will CED under TCET be any better? And why?
ChatGPT
The TCET pathway does incorporate the concept of Coverage with Evidence Development (CED) to generate additional evidence for breakthrough devices. There have been concerns about the effectiveness and impact of CED clinical trials in the past, and, the document does not explicitly address whether CED under TCET will be any better. However, the TCET pathway introduces several elements that aim to enhance the quality and relevance of evidence generated through CED.
Firstly, the TCET pathway emphasizes the importance of collaboration between CMS, the manufacturer, and other stakeholders such as the FDA and AHRQ (Agency for Healthcare Research and Quality). This collaborative approach aims to ensure that the evidence development process is well-designed, scientifically rigorous, and aligned with the needs of Medicare beneficiaries.
Secondly, the document highlights the significance of pre-specifying study protocols, including patient inclusion criteria, endpoints, treatment settings, and analytic approaches. This emphasis on clear protocol specification aims to enhance the transparency and robustness of CED studies conducted under the TCET pathway. [BQ: However, CMS concurrently released its CED plan for early Alzheimer's disease, and that CED plan meets few of the aspirational goals just mentioned.]
The TCET document encourages manufacturers to propose clinically meaningful benchmarks for each study outcome and provide supporting evidence. This suggests a focus on defining meaningful endpoints and ensuring that the generated evidence is relevant and impactful.
While the document does not guarantee that CED under the TCET pathway will be inherently better, it does reflect CMS's intention [aspiration] to improve the evidence development process and address some of the limitations associated with previous CED initiatives. By involving multiple stakeholders, emphasizing rigorous study design, and promoting meaningful endpoints, the TCET pathway seeks to enhance the credibility and impact of evidence generated through CED.
Some have accused CMS of exaggerating that its CED just implements studies of the AHRQ. Critics have said this is not true, that AHRQ has virtually no involvement with the CMS CED. [See footnote.*]
What does the new document say about AHRQ involvement in TCET and its CED.
ChatGPT
The document acknowledges the involvement of the Agency for Healthcare Research and Quality (AHRQ) in the TCET pathway and its collaboration with CMS. It states that AHRQ will review all Coverage with Evidence Development (CED) National Coverage Determinations (NCDs) established under section 1862(a)(1)(E) of the Act, which includes the TCET pathway.
According to the document, AHRQ will collaborate with CMS in evaluating the Evidence Preview and Evidence Development Plan (EDP) for each TCET candidate. The approvals for these documents will be a joint CMS-AHRQ decision, indicating AHRQ's active participation in the TCET process.
Furthermore, the document highlights that AHRQ will continue to review all CED NCDs consistent with current practice. It implies that AHRQ's involvement in the TCET pathway aligns with its role in reviewing and providing input on CED studies.
While the document does not provide extensive details on the specific nature and extent of AHRQ's involvement in TCET and its CED, it does confirm that AHRQ is a partner with CMS throughout the development and approval of the Evidence Preview and EDP.
What is the stance of CMS to breakthrough devices?
The Trump administration thought they were great. The Biden administration seemed to think in 2021 that Breakthrough Device was a not-very-meaningful designation. Can we glean how much they weight the value of Breakthrough Device here?
ChatGPT
The document does not explicitly state the stance of CMS towards breakthrough devices. However, it provides the TCET pathway as an expedited coverage option specifically designed for breakthrough devices. This suggests that CMS recognizes the potential value and importance of breakthrough devices in delivering improved outcomes to the Medicare population.
The TCET pathway aims to provide time-limited coverage for breakthrough devices that do not yet meet the reasonable and necessary standard for coverage under section 1862(a)(1)(A) of the Act. It emphasizes the need for further evidence development through the CMS and AHRQ-approved Evidence Development Plan (EDP). This indicates that CMS acknowledges the importance of generating additional evidence to support coverage decisions for breakthrough devices.
While the document does not explicitly weigh the value of breakthrough devices, the establishment of this new dedicated pathway and the emphasis on evidence development suggest that CMS recognizes the significance of these devices and their potential benefits for Medicare beneficiaries.
PRESS RELEASE / FACT SHEET
Chat GPT Summarizes the Press Release
The press release highlights the main points of the new TCET pathway for Medicare coverage of breakthrough devices. Here are the key takeaways:
- The TCET pathway aims to provide timely and predictable access to new medical technologies for Medicare beneficiaries while maintaining safeguards and promoting innovation.
- It utilizes the existing national coverage determination (NCD) and coverage with evidence development (CED) processes to expedite Medicare coverage for certain FDA-designated Breakthrough Devices.
- The TCET pathway includes an evidence development framework that allows manufacturers to address evidence gaps through fit-for-purpose studies, including the use of real-world data.
- CMS collaborated with the Agency for Healthcare Research and Quality (AHRQ) to develop a comprehensive approach that incorporates greater flexibility into the proposed CED paradigm and allows for fit-for-purpose study designs.
- The TCET pathway involves an Evidence Preview, which is a focused literature review to assess the available evidence and identify any evidence gaps. It provides transparency and informs coverage decisions.
- Manufacturers develop an Evidence Development Plan (EDP) to address evidence gaps identified in the Evidence Preview. CMS and AHRQ evaluate the EDP for scientific integrity and relevance to the Medicare population.
- The TCET pathway is specifically applicable to FDA-designated Breakthrough Devices within a Medicare benefit category that are not already covered under an existing NCD or excluded by law or regulation.
- CMS will consider nominations from manufacturers interested in participating in the TCET pathway, and the nomination process involves meetings with CMS and FDA to discuss the technology and review timing.
- The duration of coverage under the TCET pathway is tied to the CMS- and AHRQ-approved EDP, typically lasting for three to five years to generate evidence and address evidence gaps.
- There will be a transition to post-TCET coverage, where CMS will conduct an updated evidence review and consider whether to continue coverage, require further evidence development, issue a non-coverage decision, or delegate coverage decisions to Medicare Administrative Contractors (MACs).
CMS is seeking public comment on whether similar devices to the Breakthrough Device should be subject to separate coverage conditions or follow the same requirements, including proposing an EDP.
CMO's BLOG
Summary of the Blog of the Chief Medical Officer
The blog post by the Chief Medical Officer provides an overview of the proposed Transitional Coverage for Emerging Technologies (TCET) pathway and the revamped evidence development framework introduced by CMS. Here are the main points:
- The TCET pathway is a voluntary program designed to expedite Medicare coverage for certain FDA-designated Breakthrough Devices. It aims to provide an efficient, predictable, and transparent coverage review process while ensuring robust safeguards for Medicare beneficiaries.
- The TCET pathway utilizes national coverage determination (NCD) and coverage with evidence development (CED) processes to accelerate Medicare coverage for Breakthrough Devices. It includes a pre-market evaluation of potential benefits and harms of technologies and identifies any evidence gaps.
- The pathway allows manufacturers to address evidence gaps through fit-for-purpose studies, where the study design, analysis plan, and data are appropriate for answering specific research questions. Fit-for-purpose studies may leverage existing real-world data, making them more convenient for manufacturers.
- CMS may conduct an early evidence review called the Evidence Preview before FDA marketing authorization. This review helps determine the best available coverage pathways based on the strength of the evidence.
- CMS engages with manufacturers to discuss evidence gaps and potential study designs that can address them. Manufacturers can propose an Evidence Development Plan (EDP), which is developed in collaboration with CMS and considers both CMS evidence development and FDA post-market requirements.
- The goal is to finalize a TCET National Coverage Determination (NCD) within six months after FDA market authorization. Coverage under the TCET NCD will continue only as long as necessary to generate timely evidence for informed decision-making and establish long-term Medicare coverage.
- CMS sought extensive feedback from stakeholders during the development of the TCET pathway and evidence development framework. The partnership with the Agency for Healthcare Research and Quality (AHRQ) contributed to incorporating greater flexibility and fit-for-purpose study designs.
- CMS has issued proposed criteria, updated guidance documents, and published a series of guidance documents reviewing health outcomes and their meaningful differences within priority therapeutic areas. The public will have an opportunity to provide comments on these documents.
The blog emphasizes CMS's commitment to promoting access to emerging medical technologies while upholding rigorous evidence standards and ensuring the health and well-being of Medicare beneficiaries.
NEW NCD EVIDENCE GUIDEBOOK
CHATGPT Reads the Evidence Guidance Document - A Rulebook for Readoing Evidence for NCD Authors
The Evidence Guide outlines the methodological principles and criteria used by the Centers for Medicare & Medicaid Services (CMS) when evaluating clinical evidence to make national coverage determinations (NCDs) regarding the reasonable and necessary coverage of medical items and services. The guide aims to provide a framework for assessing the quality, applicability, and strength of evidence to ensure that coverage decisions are based on reliable and relevant information.
The guide emphasizes several key rules and considerations:
Methodological Principles: CMS takes into account various methodological aspects when reviewing clinical evidence, such as randomization, contemporaneous control groups, prospective studies, sample sizes, masking, and thorough documentation. The goal is to minimize bias and ensure internal validity in the study designs.
Review of Individual Studies: CMS evaluates the quality and relevance of individual studies, considering factors like risk of bias, precision of estimates, consistency in the direction of findings, and applicability/external validity. These criteria help determine the confidence and generalizability of study results.
Strength of Evidence Assessment: CMS assesses the strength of evidence across multiple dimensions, including study design, conduct, and outcomes important to patients. The guide highlights the importance of clinically meaningful and durable benefits, while considering the balance between harms and benefits. Case series and case reports are generally assigned lower evidentiary value.
The goal of the Evidence Guide is to promote a comprehensive and transparent evaluation of evidence, ensuring that CMS coverage decisions are based on rigorous and reliable information. By providing clarity on the factors considered and the evaluation process, the guide aims to support predictable and consistent coverage determinations.
It is important to be aware of the Evidence Guide because it outlines the criteria and standards used by CMS to assess the evidence supporting Medicare coverage decisions. Understanding these guidelines can help stakeholders, including healthcare providers, manufacturers, and researchers, align their evidence development strategies and submissions with CMS requirements. Being familiar with the guide can facilitate meaningful engagement in the coverage determination process and ensure that Medicare beneficiaries have access to high-quality and evidence-based medical interventions and services.
 |
| 10 pp |
Error in TCET Guide
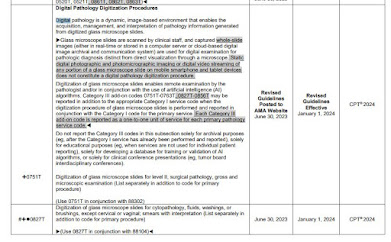
CMS makes an error in the TCET proposal which I've seen it makes before, that BT Device applies to only one unique product [sic]. In fact, FDA issues BT Device Status (a review channel) to every applicable device up until one is approved for market. For examle, similar devices A,B,C, may be granted BT status in January, February, March, and they all still are "breakthrough devices" if they are successively approved in July, August, September. More than one device in a category is allowed (for example, comparable liquid biopsy tests from both Guardant and FMI recently approved as BT devices by FDA).
__
* A general counsel of HHS under Trump wrote a memo that CMS's engagement with AHRQ was so think as to not support this leg of the legal standing the statuted required for CED. See more on GC Charrow's AHRQ legal memo and also several relevant links, in my blog here.
###
A proprietary source, Washington Analysis, headlined that the TCET "underwhelms," which I concur with.