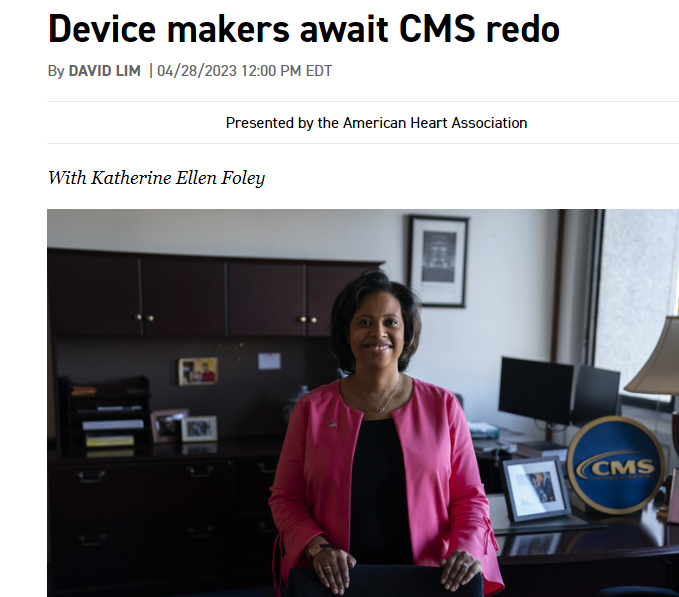
Readers will know I have enjoyed experiments with how ChatGPT4 approaches medical policy decisions and discussion.
I was wondering what happens if more and more courts take on cases that review FDA decisions, some courts asking that Drug ABC be taken off the market, and other courts ruling that Drug ABC is properly approved and shall stay on the market. Now, I am not an attorney, and GPT4 is not an attorney, so this essay is just an experiment in learning the style and approach of AI answers to legalistic questions. here we go. My human text is in blue.
You are a legal expert,
experienced in jurisprudence and judicial procedure, and especially in the
federal court system. The issue is discrepant rulings (such as injunctions), each of which is styled to
have national impact immediately, from different judges among the 700 federal
court judges.
The case study in this
hypothetical is a drug, ABC, approved by FDA in 2000.
·
Plaintiff A in
District A asks for an injunction against its sale, due to mistakes in the FDA
approval process. She wins her case with Judge A. Sales of ABC are injoined nationwide.
·
Plaintiff B in
District B asks for an injunction against the sale for its indications 2 and 3,
while expliciting rule that indication 1 is a proper agency action and shall stand. Judge B grants this national injunction.
·
Plaintiff C in
District C also asks for an injunction against all indications due to
irregularities in the FDA approval, but this plaintiff loses and the judge
rules that, as FDA requests, all indications can stay on the market.
·
Clearly, these back-and-forth
cases and stakeholders could multiply further. Turning to the appellate courts, their rulings are also contradictory (!), some staying the lower court pending
hearings, others allow the lower court national injunction to remain in effect.
The Supreme Court does not want to have an activist "shadow docket,"
yet doing nothing seems to be a road to chaos with chaotic lower court and appellate
contradictory rulings - are sales banned by injunctions, if so, which ones and
in what degree, and what is the role of a particular judge's "anti-injunction" ruling affirmatively that the FDA drug is
properly and legally approved and shall stay on the market.
Finally, as one piece of
context, in 2023, FDA-related cases of the type in this hypothetical are
actually happening. Discuss the situation from a jurisprudence perspective and
discuss possible options and outcomes.
As an AI language model, I am not a legal expert, but I can
provide some insights into this hypothetical situation involving discrepant
rulings in federal courts. This scenario presents a challenging and complex
issue that highlights the potential for conflicting decisions among lower
federal courts and the implications for the federal court system, stakeholders,
and the broader public.
1. Jurisprudence
perspective: The situation reflects the fact that federal judges can have
differing interpretations of the law, which can result in conflicting
decisions. While judges strive to apply the law consistently, they may disagree
on the application of legal principles or the weight of the evidence. In this
case, the main issue centers on the validity of the FDA's approval process for
the drug ABC and whether any mistakes or irregularities warrant an injunction
against its sale.
2. Role
of appellate courts: The appellate courts serve to review the decisions of
lower courts and provide more uniformity in the interpretation and application
of the law. However, appellate courts may also disagree with one another,
leading to further confusion and potential chaos. In this situation, it is
essential for the appellate courts to act swiftly and provide clear guidance to
the lower courts, stakeholders, and the public.
3. Role
of the Supreme Court: The Supreme Court has the ultimate authority to
resolve conflicts among the lower courts and provide a uniform interpretation
of the law. The Court may be reluctant to engage in "shadow docket"
decisions, which are rulings without full briefing or oral argument. However,
the Court may be compelled to intervene to provide clarity and prevent chaos
resulting from conflicting lower court decisions.
4. Possible
options and outcomes: a. Supreme
Court intervention: The Supreme Court could grant certiorari to one or more of
the cases and issue a decision that resolves the conflict among the lower
courts. This would provide a definitive resolution to the issue and guidance
for future cases involving similar disputes. b. En banc review: If
appellate courts issue conflicting rulings, one or more of them may choose to
rehear the case en banc, meaning before all of the judges on the court. This
can help to resolve inconsistencies and provide a more consistent
interpretation of the law. c. Congressional action: In response to the
chaos resulting from conflicting judicial decisions, Congress may choose to
pass legislation that clarifies the FDA's authority or establishes a more
explicit framework for the approval and regulation of drugs like ABC. This
could help to minimize future disputes and provide clear guidance to courts,
the FDA, and stakeholders.
In conclusion, the hypothetical situation involving
discrepant rulings on the FDA-approved drug ABC highlights the potential for
conflicts among federal courts and the challenges of achieving uniformity in
the interpretation and application of the law. Possible solutions to this issue
include intervention by the Supreme Court, en banc review by appellate courts,
or congressional action to clarify the legal framework surrounding drug
approval and regulation.
You have focused on middle to
long term events, such as a definitive SCOTUS ruling, or legislation.
What
happens in the shorter term? For example, one district rules that due to
problems at the FDA, sales are nationally banned, and another district rules
the opposite, that the drug is properly approved and shall remain on sale. What
would happen if the Supreme Court declined to act (other than to accept cases for next year's docket)?
In the short term, when conflicting district court rulings
exist, and the Supreme Court declines to act, the legal landscape can be quite
uncertain and chaotic. Here are some possible short-term outcomes:
1. Status
quo and conflicting injunctions: If the Supreme Court does not intervene,
the conflicting district court rulings will remain in place, causing confusion
and uncertainty for the parties involved. This could lead to a patchwork of
injunctions across the country, with sales of the drug being allowed in some
jurisdictions while being banned in others.
2. Stay
of injunctions: The appellate courts may issue stays of the district court
injunctions, effectively halting their enforcement until the appellate courts
reach a decision. This would maintain the status quo while the cases are under
review, but it may still create uncertainty for stakeholders and the public.
3. Consolidation
of cases: One possible solution to the conflicting rulings in the short
term could be the consolidation of the cases at the appellate level. The
appellate courts may decide to hear the cases together, either by consolidating
the appeals or coordinating their review in some manner. This could help
streamline the legal process and promote a more consistent interpretation of
the law among the appellate courts.
4. Voluntary
compliance by stakeholders: In the face of legal uncertainty, the
manufacturer of the drug or other stakeholders may choose to voluntarily
suspend sales of the drug until the legal issues are resolved. This would be a
strategic decision by the parties involved to minimize their exposure to
liability and mitigate the potential consequences of the conflicting
injunctions.
5. State-level
action: State governments or regulatory agencies may also take action in
response to the uncertainty created by the conflicting rulings. They might
impose their own restrictions or guidelines on the sale of the drug, which
could help provide some level of clarity for stakeholders and the public within
their jurisdictions.
It is essential to note that in the short term, the
situation would likely remain uncertain and potentially chaotic, as various
stakeholders navigate the conflicting rulings and legal landscape. The
ideal resolution would come from a higher authority, such as the Supreme Court
or legislative action, to provide a more definitive and uniform interpretation
of the law.
I have one last hypothetical.
In District A, stakeholder A has won a ruling from Judge A that ABC should be
banned by his injunction against the FDA.
Another stakeholder, in
District E, is distressed by this ruling. She actually enters the same case and
request in District E, but intentionally defective and poorly argued. Judge E
in District E therefore quickly rules that the FDA has acted properly and ABC
is properly on the market (creating a conflict of rulings). How does this sound
as a legal strategy for opponents of the District A ruling?
While this hypothetical scenario presents an interesting
legal strategy, it is unlikely to be an effective or advisable
approach for several reasons:
1. Forum
shopping: Intentionally filing a poorly argued case in a different district
to obtain a conflicting ruling could be seen as an attempt to manipulate the
legal system, which is frowned upon. Judges and the court system generally
disapprove of forum shopping, where a party seeks to gain an advantage by
choosing a specific court or judge likely to provide a favorable ruling.
2. Judicial
discretion: Judges have discretion in handling cases and may not
necessarily issue a ruling based on the intentional weaknesses in a party's
arguments. If a judge suspects that the case has been filed with the intention
of manipulating the outcome, they may dismiss the case, abstain from issuing a
ruling, or decide the case on other grounds.
3. Ethical
considerations: Attorneys have an ethical duty to represent their clients
zealously and in good faith. Intentionally presenting a defective or poorly
argued case could be seen as a violation of an attorney's professional
responsibility and could potentially result in sanctions or other disciplinary
actions.
4. Precedential
value: Even if the strategy were to result in a conflicting ruling, the poorly
argued case may carry less precedential weight in subsequent litigation due to
the weaknesses in the legal arguments. This could limit the impact of the
ruling and make it less persuasive in future cases.
5. Inefficiency
and costs: Pursuing this strategy would add to the workload of the courts
and contribute to an inefficient use of judicial resources. It would also
likely result in increased legal costs for the parties involved.
Instead of intentionally filing a poorly argued case,
parties opposed to the District A ruling should consider more legitimate legal
avenues to challenge the decision. These could include appealing the District A
decision, pursuing a separate case with well-reasoned arguments in a different
jurisdiction, or advocating for legislative action to address the underlying
issue. These approaches would be more likely to result in a meaningful and
lasting resolution of the conflict while adhering to the principles of the
legal system and professional ethics.
 |
| bing.com/create for "conflicting court rulings" |